Salt Water
|
NATURE'S SOUP: Salt Water |
|
 Where does the salt in the water come from? It begins on shore, as runoff
from rocks and soil pulls the various minerals from land and deposits them at
sea. Evaporation at sea then concentrates the salts and the actions of
storms, earthquakes, etc. mix the salts into solution. Animals, such as
mollusks and coral, concentrate the calcium salts and crystallizes them to form
shells and reefs.
Where does the salt in the water come from? It begins on shore, as runoff
from rocks and soil pulls the various minerals from land and deposits them at
sea. Evaporation at sea then concentrates the salts and the actions of
storms, earthquakes, etc. mix the salts into solution. Animals, such as
mollusks and coral, concentrate the calcium salts and crystallizes them to form
shells and reefs.
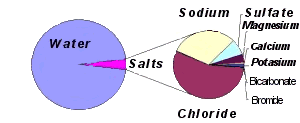
What type of salts are in the ocean? Sodium chloride (table salt), magnesium chloride, magnesium sulfate (Epsom salts), calcium sulfate (gypsum), potassium sulfate, calcium carbonate (limestone), and magnesium bromide.
(Now, in case you were wondering, sand is a mixture of pulverized rock from the tops of Georgia's mountains, transported by our alluvial rivers, plus ground up sea shells, quartz, and detritus. There is an entire branch of science dedicated to the study of organisms that live in between the grains of sand - it is called, "psalmnology".)
Just how salty is an estuary? If you remember, an estuary is the lower portion of a river, where tidal forces mix salt water from the ocean together with fresh water from the river. The amount of salt in the ocean is about 35ppt (parts per thousand), while in the estuary it is about 14-25ppt (or 1.4-2.5%). Obviously, a wide fluctuation in salinity like this makes for a big challenge to any animal living in the marsh. Some organisms, particularly many of the invertebrates, are able to allow the salinity of their bodies to fluctuate to match the surrounding water. Organisms with this type of adaptation are termed osmoconformers. Other organisms, termed osmoregulators, are able to regulate their bodies' salinity, using kidneys or a similar organ called a "nephridium", to maintain a relatively constant internal salinity .
Now, how much salt is in our bodies? About 9ppt or about one fourth the amount in ocean water. What would happen if we were stranded at sea and began to drink salt water to quench our thirst? Well, the water in our cells would drain out of our cells to try to dilute the salt in our bloodstream, through a process of osmosis. The semi-permeable membranes in our cells will only allow certain concentrations of saltiness to pass into our cells, so water drains out of our cells in an attempt to equalize the salinity. That causes us to dehydrate further. Eventually, our brains would be affected, we would go crazy, then die. The Lesson? Don't drink the water!
But the good news is, that some organism can thrive in salt water. The spartina grass, also known as marsh grass or cord grass, does quite well in salt water. Most vegetation does not. You may have noticed the trees which grew up in little island in the marsh. Those patches of trees are called locally, "hammocks" or more properly, "hummocks" (from the Indian word "hammoca" for garden place). They are growing in soil which has been deposited above the water line. Their roots are drawing fresh water, not salt water.
But there is one form of plant life in the water which thrives on the salt - the phytoplankton. Particularly, the diatoms. These one-celled algae are the star athletes of the marsh. They are nurtured on the salt and detritus in the water; and through a process of photosynthesis, they use sunlight and chlorophyll to convert the carbon dioxide into glucose and an abundant amount of oxygen.